Have a vision for your medical affairs team and business? We have a way to get you there
The pharmaceutical R&D journey for each new medicine is not only extremely expensive and risky but also very long. Billions of dollars are spent for research and development of each new medicine, that generally lasts for 10–15 years and only 1 in 10 compounds that start the clinical phase make it to the market.
Total capitalized costs for bringing new medicines to market increase at rate of 8.5% every year above general price inflation. Half of the new medicines however fail to fulfill the financial expectations.
References:
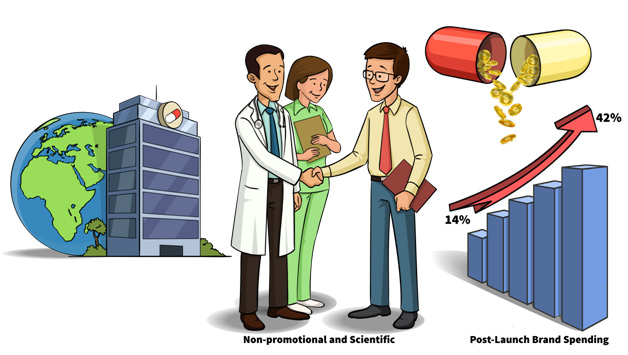
Medical affairs professionals develop various scientific communication and education strategies to bridge unmet medical needs and advance clinical practice. Strengthening and upscaling the medical affairs teams is also progressively been deployed by pharmaceutical companies for tackling the financial insecurities by credible commercialization of key products.
This can incur an average post-launch brand spending of 14% and may even rise up to 42% during the launch year initiatives.
References:
Most medical affairs professionals struggle with achieving key performance indicators synergized with commercial expectations. All their activities must be strictly non-promotional, governed by very tough internal and external regulations. Collaborative strategies for medical education and scientific communication are however crucial for success of medical affairs teams and business.
Medical affairs teams have a vital yet under-utilized role in convincing government, health authorities and policy makers to bridge health providers and patients’ knowledge gaps and update clinical practice guidelines.

References:
